I want to reply to a couple of comments that metamars made in the closed thread on Jones's Bentham paper, and I think this is probably the appropriate place to do it. I was away over Easter, so it took a while to catch up, but the specific post is at
http://www.internationalskeptics.com/forums/showthread.php?postid=4599311#post4599311.
First of all, I think this may have been covered, but I'd like to put it to bed:
No, I have it the right way round. The Wikipedia article on energy density (
http://en.wikipedia.org/wiki/Energy_density) explains it similarly:
Therefore thermite, which holds its own oxidiser, has a lower energy density, at around 4kJ/g, than combustible materials that don't contain their own oxidisers, for example kerosene at around 43kJ/g.
Now the rather more complex point:
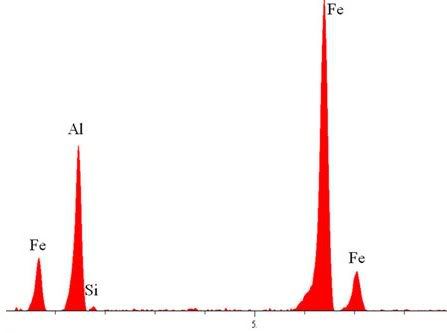
Let's take a look at the figure in question, reproduced here under fair use conditions as a small part of the above referenced work for research.
[URL]http://www.internationalskeptics.com/forums/imagehosting/1476449e7178ebe28d.bmp[/URL]
It's not entirely clear, but look at the Y axis. The highest value quoted is
less than 4000J/g, the figure for the energy density of thermite.
Now, let's address the comment "Apparently you're thinking of perfect reactions". I'm using the term "energy density", which is perfectly clear, and is equal to the amount of energy that can be released in a 100% efficient reaction. What we can see from Grainier's thesis is that the amount of energy released by any thermite formulation is less than its energy density, but that the efficiency of energy release is greater for smaller particle sizes. This is completely consistent with the fact that the energy density of thermite is independent of particle size, as I originally pointed out.
Now, going back to the paper, DSC results indicate energy releases of 1.5, 3, 6 and 7.5kJ/g from four samples, with the comment made that the underlying grey layer is included in the mass but does not react, so does not contribute to the heating. We can assume, therefore, that the minimum possible value of energy density of the material believed to be thermite is 7.5kJ/g, or nearly twice the energy density of pure thermite. This is assuming a perfect reaction, and assuming that there is no inert grey layer on the sample giving the highest energy release; if either of these assumptions is invalid, then the actual energy density must be greater. The conclusion is simple and inescapable: the material
is not thermite...
Dave