What I think I should have asked for initially is for someone to go through all the numbered tests in the opening post and predict what the result would be and why under 2 different sets of conditions:
Condition 1: If the chips were not some form of thermite
Condition 2: If the chips were some form of thermite
That's what I was trying to understand.
I think the most appropriate question about the test proposal would be: What questions is the test supposed to answer, and are the methods appropriate to answer that question and leave as little doubt as possible? Is the study well-designed?
I am not sure what Basile's question actually is.
Now on to your request: I propose that we look at two competing hypotheses. Your condition 1 isn't well defined; it is hard to make specific predictions for "anything else but".
So allow me to make predictions for the two main Hypotheses:
A. Each chips represents one of several red primer paints on spalled, oxidized structural steel
B. Each chip represents some preparation of nano-thermite of the Al+Fe2O3 persuasion, mixed with some other, yet to be determined matrix materials
1. - Red/gray chip separation using optical microscopy and magnetic attraction to assist in isolation of particles of interest.
These (and none other, except of course that the source material is dust collected very near GZ very soon after the tower collapses) are the two selection criteria that Harrit e.al. describe in their study to determine which particles to study and which to leave out.
This begs the question if all particles thusly selected are the same material. Harrit e.al. are utterly unclear on this question: On the one hand, they talk about at least 6 different kinds of such red-gray chips in their study (distinguishable by presence or absence of significant chemical elements in either the red or the gray layer), but treat them as if they were still all basically the same material. They claim that all such chips, whatever their detailed make-up, react thermitically, and all have properties different from (some arbitrary, unidentified) paint. But when James Millette followed the same selection procedure and proved that there are magnetic and red-gray chips, that even match Harrit's "main" specimens (Fig. 2-11) in great detail of elemental composition and microscopic structure, that definitely contain no thermite at all and instead contain nothing but ingredients that are universally typical for primer paint, the Harrit-Team accused him of selcting the wrong chips, demanding that he use further selcection criteria, which Harrit e.al. themselves did not employ consistently, if at all.
So I predict that
A. If Basile's new study shows
no thermite, Harrit and colleagues will say he used the
wrong selection method
B. If Basile's new study shows paint, Harrit and colleagues will say he used the
correct selection method
The difference in result is one of consistency with expectation.
2. - Optical images of collected particulates as collected at appropriate magnifications to record condition as collected.
In either case, particles will look brightly, clearly red just like paint, and gray and shiny, just like oxidized steel.
This method would do nothing to differentiate between the hypotheses. However, I guess it is good practice to show your specimens.
3. - SEM/EDX with elemental quantification of red/gray chips, both red and gray layers.
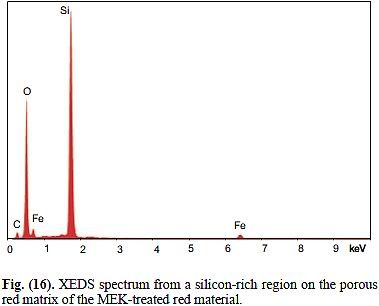
A. Element quantities will be consistent with common organic binders (mainly C and O in the EDX spectra) making up >50% of the mass of the chips; iron oxide (Fe2O3) making up a substantial proportion of the rest, but less than 30% of the total mass; elements such as Si, Ca, Al that are common in many minerals commonly used in paints as cheap filler; and lesser amounts of elements typical for corrosion-inhibiting salts, such as Cr, Zn, Pb, Sr.
In any case, there will be a LOT more Fe than Al, if there is Al at all. If there is Al, it will be paired with equal or higher amounts of Si and/or Ca.
B. Fe and Al should be present in 1:1 molar proportions or very close to it. Both should make up >50% of the mass of the red layer, while organic materials (C, O) should be far under 30%.
4. FTIR analysis of organic components of red/gray chips, both red and gray layers.
Would not be certain to differentiate the hypotheses, as for example epoxy, which can be identified with FTIR, has been described as the matrix material for both steel primers and thermite compositions. However, some organic components would be highly typical for paints and have very little use in energetic materials (e.g. hardened oils), or vice versa. The possibilities are manyfold, can't predict them all.
5. ESCA small spot technique with argon ion sputter for depth profiling to definitively establish the presence of elemental aluminum within the red layer of the red/gray chips. Scans of gray layer also to be taken to add to information base.
I don't know / understand that method, so I don't know if it really does competently what Basile wants to use it for.
IF that technique can in fact identify or rule out elemental Al, then of course...
A. Find no elemental Al
B. Find lots of elemental Al
6. DSC analysis of red/gray chips focusing on exothermic/endothermic reactions near 400 degrees C. Some chips to be scanned in inert atmosphere and some in air or oxygen containing gas stream.
First of all, I'd demand that the red layer be separated from the gray layer, and both be tested in separation. They way Harrit e.al. did their DSC tests, they KNEW they had (on average) >50% by mass chemically inert gray mass, that nonetheless might show some thermal reactions (phase changes). It makes it unnecessarily difficult to interprete the results if you don't separate materials.
Doing the DSC-test under air, knowing that the red layer contains significant amounts of organic matrix, is a near useless excercise:
A. As there are so many different kinds of paint with a number of different organic binders in varying amounts, it is near impossible to predict shape, position and features of the DSC-traces; except of course that almost all organic materials release a lot more energy than any thermit composition.
B. Truthers find more and more papers where energetic nano-materials are developed and tested - and they show peaks and curves and ignition temperatures all over the place.
It follows: You can't tell for certain from the DSC-data what class of materials you are testing.
7. SEM/EDX with elemental quantification of residual products of DSC analysis of red/gray chips.
Would not help much to differentiate the hypotheses. In both materials, organic binder would decompose and burn - and what else happens is near impossible to predict.
Basically, organic material would largely vaporized, i.e. C and O would be depleted in the solid reaction products, but metals would still be there in almost the same proportions as before.
SEM/EDX is not a very good method to identify chemical compounds or tell them apart from elements.
8. Optical images of reaction products after DSC experiments.
Would not be of great help
A. You'd expect the iron oxide to not react at all, so unless charring is heavy, you'd still see red material.
B. The red color should largely or completely disappear, as the red pigments (Fe2O3) are supposed to react and thus "disappear" (turn into something else - elemental iron).
Basile ought to add at least one other method to directly and unequivocally identify compounds; P-XRD is an excellent tool, almost all studies on nanothermite that truthers like to cite use it. Basile should most definitely look for, and quantify, all of the following both before and after burning samples:
Al
Al-oxide
Fe
Fe-oxide
Only if you find significant Al + Fe-oxide BEFORE the reaction, little to none of both AFTER, but instead find lots of Al-oxide + Fe AFTER and none or little BEFORE, can youi be sure that the thermite reaction actually happened.
P-XRD can identify all four substances for you. Steven Jones did it, but never published results.
Of course, you'd not predict much Al in any paint, and you'd not predict that Al2O3 is found in paint only after burning.
Another good method to identify individual particles of metals and metal oxides is TEM-SAED. Jeff Farrer did it, but never published results.