Unlike you, I am reserving judgement on this.
OK peeps lets do some science. Lets first start by reading the paper and doing some analysis on the results specifically with regard to the aluminium.
The first thing is to look at Fig 10 because this Fig is an XEDS map of the various elements across an area of the sample. What this does is helps to visualise where the different elements are and whether there is any connection between them. Humans are good at spotting patterns and the best way is to slowly move your head back from the screen as you look at the graphic. Remember we are interested in Al so concentrate on the purple square. Now you are back a few feet look at the other squares and compare the intensity to the purple square. Notice anything?
You did didn't you? What you noticed was that the turquoise and purple squares correlate extremely well. Especially noticeable is the diagonal / in the centre. This means that whatever the turquoise square represents, in this case Silicon (Si), it is strongly connected with Aluminium. In the materials scientists head cogs are starting to turn, because this information pushes us into a certain direction.
So lets now compare the Al and Si maps with the other elements. Fe doesn't show any correlation, infact if anything it's the opposite. We are now thinking that whatever contains the Al and Si doesn't contain much Fe. Onto the O. Again we see correlation with the diagonal and some other points (including the dark vertical area to the left of the diagonal), but we don't see such a striking correlation as with Si and Al, however the correlation is there. Oxygen is found in lots of different compounds and will therefore be associated with parts where Al and Si aren't found in the map. What's important is that there is correlation. Finally Carbon (red square) doesn't match with Al or Si, it doesn't look like it tallies much with Fe, but there is correlation with O. This is quite expected. So now what do we do?
Well you can see the grey BSE image (a) above which is the region where the XEDS map was produced. Look at that image and then look at the Al and Si maps. What do you see? Yep, it's that diagonal again. This means that the Al, Si and O in that diagonal are all associated with that particle. Also not the particle that is just to the right and below the (a). Again that particle has similar associations.
We can also note that the clumps of white particles in that BSE image match that of the Fe map. It's safe to conclude that we have distinctly separate particles, one containing Fe and the other containing Al,Si and O.
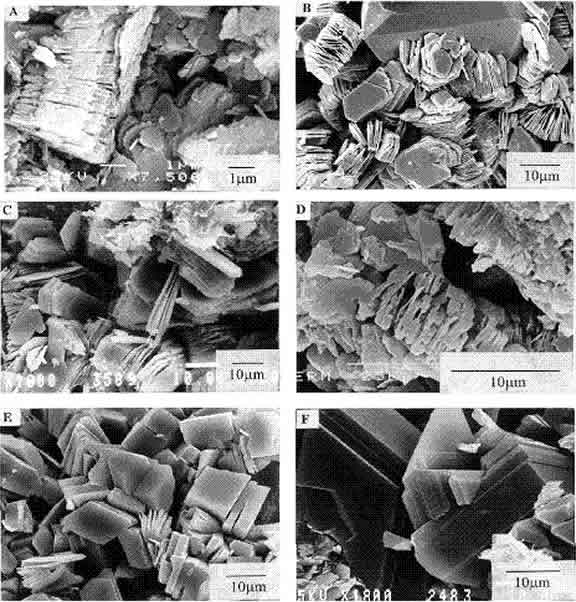
So lets take a look at the SEM photo-micrographs.
In the middle of photo (a) we can clearly see these particles. Looking at the other photos it becomes self evident that these particles are thin sheet-like particles (platelets) and they have an hexagonal shape to them (d). There is also something else that is interesting about these platelets. Look at the arrow in photo (d). Look at the platelets.
What's happened to them? They are all stacked up together. Why is that? What causes that?
So now we have established that the Aluminium and the Silicon as well as some of the Oxygen in the sample (of the red layer) is directly linked to these thin hexagonal platelets.
Has anyone ever had a grow your own crystal toy as a child? You get a tiny seed and place it in a solution and the seed grows and forms a shape. Different solutions give you different shapes. Well the exact same thing happens with platelets like these and also with the whitish grains you can see in the same SEM photos. These whitish crystals are rhomboidal (some say rhombohedral) in shape and because Fe and O are associated with them then we are sure that they are Fe
2O
3 - experience and reference shows us that this is correct.
Closer magnification as seen in Fig 9 - shows us to be correct.
We also notice that there is another material present, namely a Carbon matrix. So now we know that Al cannot be free or elemental, it is bound with Si and O in the crystal. If it wasn't bound in the crystal then we would expect to see another particle that was of a different morphology (structure).
So what are these hexagonal platelets that contain Al, Si and O. These elements point us in the direction of Aluminosilicates, which are minerals or clays, but which one?
Well we could be here for days trying to find the right one. But do you remember the strange stacking? The thin hexagonal platelet. Yep, they are odd aren't they? Kind of stick in the mind as a feature that if you ever came across it again you'd think, "hey, I've seen that!".
Does this remind you of anything?
Or this? (Below are Scanning Electron Microscope Pictures of Kaolinite Interstratified with Illite.
Note the platelets of Kaolin are easily observed. )
http://www.smianalytical.com/clay-analysis.html
and how about this?
Remember the XEDS maps and how the Si is associated with Al, remember the identical peak ratios for the platelet
Kaolin (Kaolinite) Al
2Si
2O
5(OH)4 will give identical Al and Si peaks, it also has the same shape (as proven above), it also stacks just like the platelets are stacked in Jones' samples. I don't know of any other aluminosilicate material that stacks or has this shape. Does anyone else? Metamars, can you find a material that has these characteristics, but isn't kaolin? Kaolin is a
natural mineral. It's morphology is thin platelets.
There is no man made engineering going on, just mother nature some physics and chemistry. Wow, how cool is that, material that are nanometres thin are found naturally occurring in minerals.
Kaolin is used in thousands of materials. There is nothing odd or strange about it (except perhaps it's shape and the natural way it's platelets stack - which gave the game away). And there is no elemental aluminium in Kaoulin.
So what happens to the thermite reaction if there is no aluminium free to oxidise? Yep, that's correct, the thermite reaction cannot take place. This proves that the samples are not thermite and did not undergo a thermite reaction in the DSC. Something else reacted and because air was present then combustion most likely took place.
And that folks is how science is done.
Note that no one complains about elemental Oxygen. No one tries to say that the Oxygen isn't part of a compound with the Iron. Yet that is exactly what they do when they talk about the Aluminium. The data in the paper proves Fe2O3 crystals are present and the same data proves that Aluminium is bound up in platelets of Kaolin, so why is no one having a go at elemental O? Why just the Aluminium?
The paper also specifies an MEK test on sample to free this elemental Al, but it isn't any of the samples a,b,c,d that had the full analysis and DSC tests conducted. Infact it's another material altogether.
.068 * 1000 nm = 68 nm. I find the platelets to be 68 nm thick, give or take. How thick do you determine the platelets to be?
Yes, that looks about right. No-one ever measures particle size via their thickness. It's always across the largest diameter.
Imaginary experiment - imagine we take a whole range of diameters of hexagonal platelets, but their thickness doesn't change at 68nm. Now if we pass them through a mesh what is the determining factor for whether they are trapped in the mesh or fall through assuming the mesh hole is round?
That's right it's the platelet's diameter that determines whether it's collected or not. So we characterise materials via their diameter. In any case natural materials produced in mother nature are formed as crystals and do form at very small scales as I have shown.