R Mackey, thanks for a pedagogical answer that makes total sense.
1. One thing was new to me. You said that the "magnetic nature" was inconsistent with nanothermite if I understand you correctly. Would you explain that. Would nano not be magnetic, or... ?
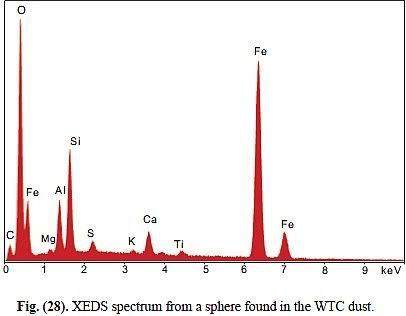
2. About the presence of Al and/or Si, does the relative amount of each compared to Fe and O give a hint of what the substance is or what is it is not?
3. Chris Mohr's experiment I am not familiar with. Is that mentioned here in the forums?
4. Are you or either persons in here professionals within chemistry or would I have had the same knowledge as you if I had chemistry in high school?
5. About the issue of Harrit et al. not testing in an inert atmosphere, I went through the report again, and I could not determine what kind of atmosphere he tested in at all. So how do we know for sure that he did not test it in an inert atmosphere? Can we conclude that solely from him not mentioning it?
Again, I really appreciate everyone here, having patience with my questions. I need to get facts straight for our website before cocluding anything and in order to confront Harrit properly and with the relevant questions.
Cheers
Steen
Hi Steen! Welcome to the Forum!
Disclosure to all others: Steen and I have privately chatted about the Harrit e.al. paper about 2 months ago.
To chime in with some answers:
1. I fail to see R.Mackey's point - owing perhaps to my poor understanding of magnetism. Thermite (whether nano or not) contains iron oxide. It is generally assumed that this would be Fe2O3 in its alpha-phase which is
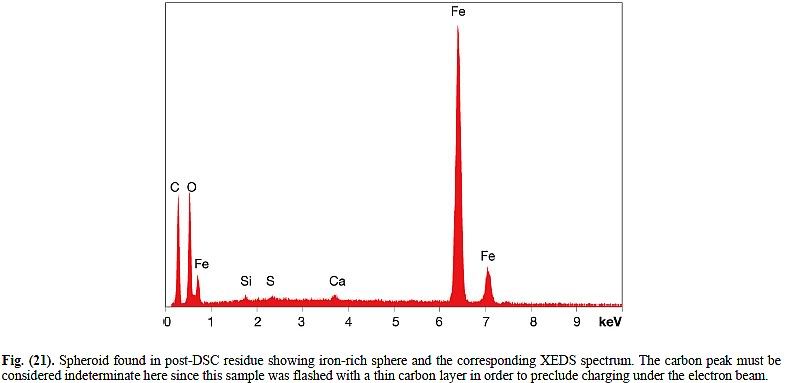
weakly antiferromagnetic, but there is no principal reason why other iron oxides couldn't also be a part of it. Jones's red-gray chips are most likely paint on spalled, oxidized steel, so again we'd expect alpha-Fe2O3 to dominate, but would also expect other phases (Fe3O4, FeO...) with different magnetic properties. The paint that Harrit e.al. present in their Fig. 2-11 contains alpha-Fe2O3 (rhombohedric hematite crystals are visible) below micron-size. So in terms of chemistry, crytalization and magnetism, the iron oxides in their chips is not really distinguishable from the iron oxides in generic thermite.
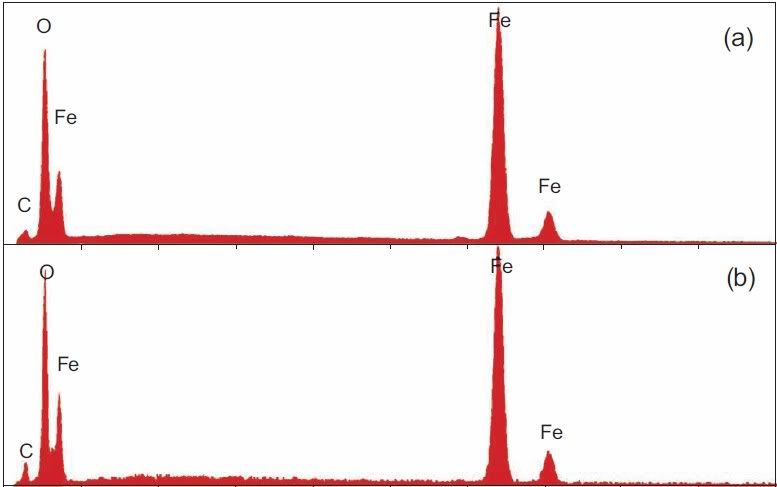
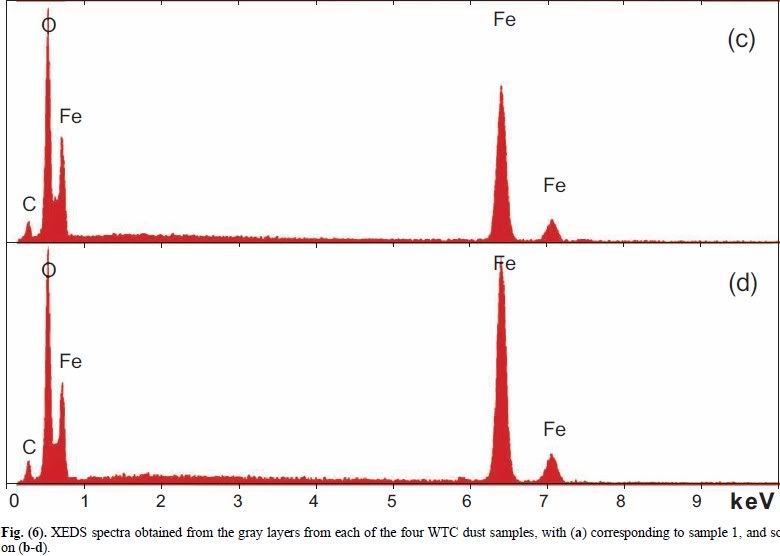
2. Absolutely yes. Fig. 7 in Harrit e.al. consistently shows Si peaks that are slightly higher than the Al peaks, Since Si also has a slightly higher atomic weight, this means that in all 4 chips (a)-(d), there is practically the same number of Al and and Si atoms. Fig 10 shows an XEDS map of several element. It reveals no particular correlation of C and O to anything else, but that Al and Si map each other very well. Also, we can see that areas rich in Al and Si correlate with those regions where we see these peculiar platelets in the BSE image. Fig 11 finally reveals that the thin platelets indeed have this signature of Al=Si but no Fe, along with lots of O, whereas Fe is assocuated mainly with O, and all other elements are considered by the authors to be the effect of a beam not focussed enough. Taken together, it would seem that Al and Si are bound with each other and O - i.e. we are looking at an aluminium silicate with equal amounts of Si and Al. Kaolinite happens to be such an aluminium silicate: it has the sum formula Al
2Si
2O
5(OH)
4. And surprise surprise: Kaolinite not only has a chemical composition that matches Harrit's XEDS data perfectly, it also looks exactly like, and has the same typical size as, the platelets and stacks of platelets that Harrit sees, too, in Figures 5, 8, 9 and 10.
(Note: C is always present in the XEDS data; that's because the pigments are, as Harrit correctly comments, embedded in an organic matrix that is just everywhere in the samples and around the pigments; H can't be detected by XEDS, that's why it is absent fromn the spectra)
3. Yes. Chris used "my" thread "Origin of the paint..." to inform us about the progress of his efforts to have red-gray chips independently tested by a competent lab. He finally decided on a lab in Atlanta Georgia and an experimenter by the name of Jim Milette. Jim has access to dust samples that were collected east of the WTC a few days after the collapses, and that were already the subject of a paper about environmental hazards, that Jim co-authored in 2002. The focus back then was on substances that may pose a health hazard, such as asbestos. Since that study wasn't interested in how the towers collapsed, they did not go into any details about, for example, particles of iron oxide or paint, simply because neither is a significant hazard.
In that thread, Chris also organized financial support - the test will cost US$1000.
Chris Mohr will give Jim Milette the go-ahead this week, after hopefully the last checks are in to add up to those 1000 dollars.
4. The Almond and Sunstealer have both extensive professional experience with the kind of material analysis of the kind that Harrit e.al did, but both are not really active here any longer. I believe they still read our threads now and then.
Ivan Kminek, I understand, is a chemist, or chemistry student, at a university in thhe Czech Republik. His background I believe is more organic chemistry - polymers and such stuff, not so much material physics or the anorganic chemistry of pigments.
All others, myself included, I believe are more or less amateurs with regard to chemistry, although some may have had a chemistry course or two at university as part of their science or engineering education.
5. I don't have a link handy, and am too lazy to search now, but we have read a reply by Harrit where he defended the choice of doing the DSC under air with the (moronic) observation that the towers weren't CDed under Argon. Note however that the DSC results that went into their paper were obtained by Jeff Farrer, probably at or near Brigham Young University in Utah, where he is a university lab manager. Note too that Jeff admitted in his AE911T interview that he had never worked with a DSC before and "quickly learned" it fromn someone right before running his tests. I don't remember if Jeff is on record with regard to the atmosphere he used.
I think it can be construed from the paper that they did the DSC test with atmospheric oxygen present. On p. 28: "
One possibility is that the organic material in the red layer is itself energetic". The usual mode for organic materials to be "energetic" is for them to burn with external oxygen. The exception are monomolecular substances that make conventional high explosives - a possibility they surely would have mentioned in that context if they knew that no extra O was present.