I'm not sure if the comment on the below link are of any interest to anyone ?
http://911blogger.com/node/13090
Could be good for a laugh anyway ?
http://911blogger.com/node/13090
Could be good for a laugh anyway ?
"Dr. Millette does not address those DSC findings which argue against the credibility of his own findings.
He shows his ignorance of the DSC work performed by the research scientists, when he states that only the "thermal properties" of a substance are revealed.
Dr. Millette ignores the importance of the findings from the DSC post ignition red chip residue.
And sadly, he shows little curiosity."
"...At the very least, it is unnecessary to burn chips specifically in a DSC:..."
"The red chips of interest, were not 'burned' in the DSC.
They were ignited in the DSC.
As you also know, the authors acknowledge and discuss in their report, the exceptional energy release when the red chips were ignited.
It would not suffice to "burn the chips" at the 400C Dr. Millette chose and then dig through the residue.
From my reading of the Bentham Paper, iron-rich spheres were always found in the residue produced by ignited red chips.
If Dr. Millette had heated the chips to at least 500C, the 430C threshold issue would at least be dealt with.
It would be expected that upon microscopic examination of the residue at least some of his [Dr. Millette] red chips would have ignited and produced iron-rich spheres, where previously none existed."
"MM, you completely and utterly dodged my question. What will a DSC of Millette's chips show besides "thermal properties"?"
"The DSC produces valuable residue.
The residue supports a thermitic reaction."
"Putting aside the "burned" vs "ignited" question. a mor4e impprrtant point here is that MM and for that matter Steven Jones claim that iron-rich spheres were created in the burning of the chips in the Bentham paper where none were found before. The Bentham authors have admitted that the energy release is too high for thermite alone. The valuable residue does NOT include large amounts of aluminum oxide. So the value of the residue is now down to the existence of iron-rich spheres after the burning/igniting of the chips that Jones et al did. Am I right MM? I am asking you because I am not sure that in recent posts here at least the issue of the iron-rich spheres emerging FROM THIS EXPERIMENT has really been addressed. So let's address it now. MM and Steven Jones seem to be saying that these iron-rich spheres are evidence of thermite (due to 2700-degree temps allegedly required to create them), which Millette is ignoring this by not doing a DSC test. What are other explanations of why burning paint chips can also create iron-rich microspheres IN THIS EXPERIMENT?"
Harrit et al said:"Several paint samples were also tested and in each case, the paint sample was immediately reduced to fragile ashes by the hot flame. This was not the case, however, with any of the red/gray chips from the World Trade Center dust."
You have been told numerous times why Dr Millette chose 400°C yet every time you refuse to acknowledge the reason even when that reason is specifically written by Dr Millette himself.It would not suffice to "burn the chips" at the 400C Dr. Millette chose and then dig through the residue.
From my reading of the Bentham Paper, iron-rich spheres were always found in the residue produced by ignited red chips.
If Dr. Millette had heated the chips to at least 500C, the 430C threshold issue would at least be dealt with.
It would be expected that upon microscopic examination of the residue at least some of his [Dr. Millette] red chips would have ignited and produced iron-rich spheres, where previously none existed.
MM
And there it is again for you to conveniently ignore.Low-temperature ashing (LTA) is an alternative to using solvents to extract inorganic constituents from an organic film or coating.6 LTA of the chips of interest was done using an SPI Plasma Prep II plasma asher. LTA was performed for time periods of
30 minutes to 1 hour depending on the size of the chip. The gray layer remained intact and the red layer residue was collected in clean water and drops of the suspension were placed on carbon-film TEM grids. After drying, the particulate was analyzed using a Philips CM120 TEM capable of SAED and equipped with an Oxford EDS system.
Chips of interest were ashed in a muffle furnace using a NEY Temperature Programmable furnace operated at 400oC for 1 hour. The gray layer remained intact and the red layer residue was prepared as described above and analyzed using a Philips CM120 TEM-SAED-EDS.
...
If heating the suspect red chips...
"The red chips of interest, were not 'burned' in the DSC.
They were ignited in the DSC.
As you also know, the authors acknowledge and discuss in their report, the exceptional energy release when the red chips were ignited.
It would not suffice to "burn the chips" at the 400C Dr. Millette chose and then dig through the residue.
From my reading of the Bentham Paper, iron-rich spheres were always found in the residue produced by ignited red chips.
If Dr. Millette had heated the chips to at least 500C, the 430C threshold issue would at least be dealt with.
It would be expected that upon microscopic examination of the residue at least some of his [Dr. Millette] red chips would have ignited and produced iron-rich spheres, where previously none existed."
"…There is absolutely no point in heating samples to satisfy your need for microspheres when you totally ignore the FTIR and TEM-SAED results…"
And there will never be any discussion until the occurrence of iron-rich microspheres is explained.
The existence of other microsphere mixes is not in question.
But evidence that shows ignited red chips produced iron-melting temperatures cannot be ignored.
MM
Hey gang,Ok, let's not ignore the iron-rich microspheres (which are a common byproduct of combustion of mixed mineral/organic materials and perfectly ordinary in many types of ashes) and study thermitic red-gray chips.
Not non-thermitic red-gray chips.
Thermitic red-gray chips.
YOU said you agree with Frank Legge that the "how-to" for selecting such chips is all in the Bentham paper.
Do you think it is still necessary to contact the authors about it?
"And there will never be any discussion until the occurrence of iron-rich microspheres is explained.
The existence of other microsphere mixes is not in question.
But evidence that shows ignited red chips produced iron-melting temperatures cannot be ignored."
"Ok, let's not ignore the iron-rich microspheres (which are a common byproduct of combustion of mixed mineral/organic materials and perfectly ordinary in many types of ashes) and study thermitic red-gray chips...."
Please provide some examples of materials you believe should have commonly existed in the dust of the WTC, that would be expected to ignite around 430C.
Materials, which upon ignition, rapidly generate temperatures in excess of the 1535C producing molten iron, which cools into iron-rich microspheres.
MM
Please...
You have absolutely zero evidence suggesting that the chips produced temperatures in excess of 1535 degrees C. Your "iron-rich micro spheres" are perfectly capable of being artifacts of temperatures much lower than that.
Please provide some examples of materials you believe should have commonly existed in the WTC dust, that would be expected to ignite around 430C.
MM

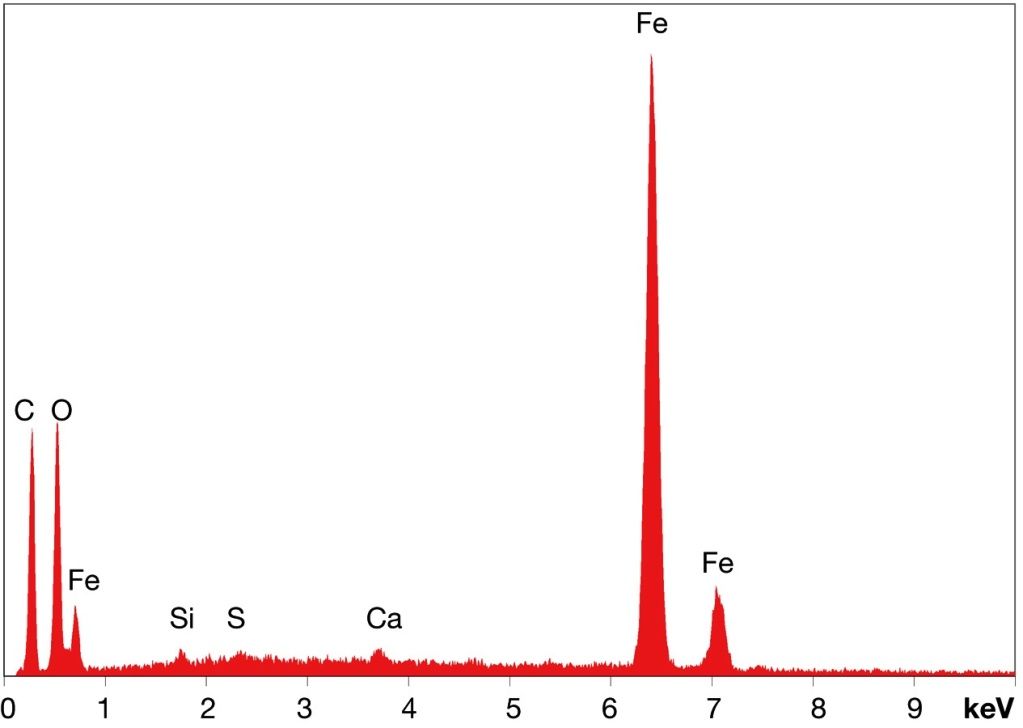
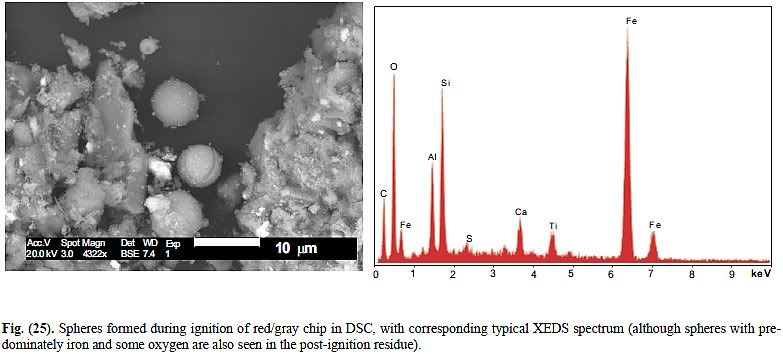
Well, in the DSC residue shown in Fig 25 I "see no indication of strontium (Sr) or lead (Pb) in his samples, but he does report titanium (Ti)". Thus, this sample does not appear to be the same material as what they reported on elsewhere in the paper.ProfJones said:I (Dr. Jones) have searched Millette's plots and see no indication of strontium (Sr) or lead (Pb) in his samples, but he does report titanium (Ti) which we do not see. Thus, his samples do not appear to be the same material as what we reported on.
"Hey gang,
On behalf of Jim Millette, I DID contact Kevin Ryan and asked him for samples of the red-0grey chips they found and reported on in the Bentham paper. Jim Millette wanted the chips but was denied them. As Jim reported in his preliminary paper, when he was unable to obtain samples from Ryan/Jones/Harrit et al he followed their protocol and found the same kind of chips in the WTC dust himself. MM it is unfair for you to blame Millette for Ryan's refusal to cooperate. You can justify Ryan's refusal but at least dona't accuse Millette of going forth with his own samples when that's all he could get his hands on. And BTW I believe the protocol for finding those chips was clearly explained in the Bentham paper and Millette was successful in finding the same kind of samples... and many 9/11 Truth people agree that Millette did indeed find the same red-grey chips."
...
He also followed a methodology which appeared to duplicate the original study by Dr. Harrit et al only so far as the red chip separation and some preliminary microscopic analysis. ...
"Please provide some examples of materials you believe should have commonly existed in the dust of the WTC, that would be expected to ignite around 430C.
Materials, which upon ignition, rapidly generate temperatures in excess of the 1535C producing molten iron, which cools into iron-rich microspheres.
MM"
"HeheIncredible
MM: clear, perfect, typical, "archetypal", undisputable, absolutely ideal examples of materials commonly existed in the WTC dust, that would be expected to ignite around 430C, are.......(surprise):
Paints …"
