Hi Oystein
You said: "You are correct that surely the iron would oxidize fast, but I doubt it would oxidize completely."
My point was if it would oxidize enough to not be disinguishable from any iron oxide sphere.
But I guess from Ivans answer that even elemental iron residue from an actual thermitic reaction is difficult to distinguish from other iron oxide spheres.
Thus, it is a much better proof to perform the reaction in an inert gas.
Kindly,
Steen
Absolutely, and of course.
Don't forget that in order to prove that iron was produced, you must have first shown that no iron was present - difficult to do when you don't analyse your gray iron/iron oxide layer and always have it present
Don't forget that in order to prove that any produced iron came from thermite reaction, you must also show that Al turned into Al oxide, and rule out other reactions.
Don't forget that more than half the mass of the red layer is organic matrix, with millions of possible reactions occurring.
So even if you manage to prove elemental iron, you haven't proven thermite yet.
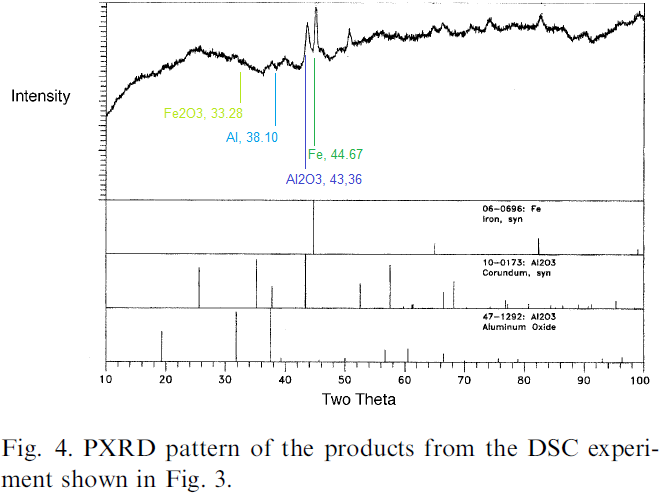
By the way: Now that Ivan linked to the Tillotson and Gash paper, take a close look at its Fig. 4 - the PXRD result:
I inserted four values for the four interesting compounds, which I retrieved from the following XRD database:
http://webmineral.com/MySQL/xray.php
Type "Fe", "Al", "Fe2O3", "Al2O3" into the search field "Element", and look for the value in round parentheses in the first column of the search result. That's the main value for "Two Theta" that's plotted on the x-axis in Figure 4.
As you can see, the peaks at Two Theta for Fe and Al2O3 are very prominent (although the graph seems shifted right by 2 or three pixels), but there is nothing at the Two Theta values for Al and Fe2O3.
So this shows that not only did Tillotson and Gash find the expected thermite reaction products, it also shows that the reactants are mostly gone. Remember T&G knew exactly that their material contained 90% thermite (ca. 23% Al, ca. 67% Fe2O3, if they aimed at a stoichiometric mix - the rest organic residue of the gel process), so there is their proof that thermite changed into thermite products - all four substances are accounted for.
Contrast this to Harrit e.al. who
- did not prove metallic Al before the DSC test
- did not prove Al2O3 after the DSC test
- did not prove absence of metallic Fe before the DSC test
- only have vague evidence of some possible metallic Fe after the reaction
All they have is some proportion (of unknown quantitiy) of iron oxide before the test - AND they also have some proportion (again, of unknown quantity) of iron oxide after the test, so they
- did not prove that Fe2O3 was reduced during the test
Steen, it's good that you try to understand fine details, and what difference different test protocols would make. But there is so much more that they would have to change about their test designe to make definitive about thermite or no thermite!