Oystein
Penultimate Amazing
- Joined
- Dec 9, 2009
- Messages
- 18,903
In my blog, Oystein's 9/11 debates, I just posted a lengthy article that exlains Basile's quantification of his XEDS data and why that proves his lucky chip #13 is not thermitic by nature:
How Mark Basile confirms that red-gray chips are not thermitic
Beware: It contains a lot of math
A discussion of this:
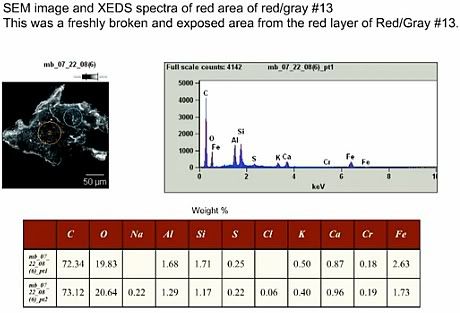
I fine-tuned the numbers from previous posts. For example, the elements add up to 100% there, but hydrogen is missing, because it can't be detected by XEDS. Hydrocarbons have typically at least 1 H-atom per C-atom, or 1 part by weight hydrogen for 12 parts carbon. So since there is 72% carbon, you'd have to add at least 6% hydrogen, bringing the total up to 106% or more. Conversely, adding an appropriate amount of H has the effect of reducing all other weight-%s by a factor of 100/106.
The low amount of 2.63% iron in Basile's samples means that at most 4.74% of his red layer could possibly thermite. Since most hydrocarbons have an energy density of at least 15 kJ/g, almost four times that of thermite, and since there is more than 18 times as much hydrocarbon in the chips than thermite, this results in at most 1.4% (but more realistally less than 1%(*)) of the heat of reaction coming from thermite when you burn the chip.
In other words: almost all(*) the heat comes from hydrocarbon combustion(**) - Basile proves it.
If, as the thread title claims, "Chemical Engineer Mark Basile confirms Harrit nano-thermite results", then all he does is confirm that Harrit's red-gray chips, too, are not thermitic.
We knew that all along, but it's nice to have it confirmed
ETA Footnote:
(*) "<1%" of course includes the possibility of "=0%". Similarly, "almost all" is meant to imply the possibility of "fully all".
(**) And possibly further reactions other than the thermite reaction
How Mark Basile confirms that red-gray chips are not thermitic
Beware: It contains a lot of math
A discussion of this:

I fine-tuned the numbers from previous posts. For example, the elements add up to 100% there, but hydrogen is missing, because it can't be detected by XEDS. Hydrocarbons have typically at least 1 H-atom per C-atom, or 1 part by weight hydrogen for 12 parts carbon. So since there is 72% carbon, you'd have to add at least 6% hydrogen, bringing the total up to 106% or more. Conversely, adding an appropriate amount of H has the effect of reducing all other weight-%s by a factor of 100/106.
The low amount of 2.63% iron in Basile's samples means that at most 4.74% of his red layer could possibly thermite. Since most hydrocarbons have an energy density of at least 15 kJ/g, almost four times that of thermite, and since there is more than 18 times as much hydrocarbon in the chips than thermite, this results in at most 1.4% (but more realistally less than 1%(*)) of the heat of reaction coming from thermite when you burn the chip.
In other words: almost all(*) the heat comes from hydrocarbon combustion(**) - Basile proves it.
If, as the thread title claims, "Chemical Engineer Mark Basile confirms Harrit nano-thermite results", then all he does is confirm that Harrit's red-gray chips, too, are not thermitic.
We knew that all along, but it's nice to have it confirmed
ETA Footnote:
(*) "<1%" of course includes the possibility of "=0%". Similarly, "almost all" is meant to imply the possibility of "fully all".
(**) And possibly further reactions other than the thermite reaction
Last edited:
