To Oystein, Chris Mohr and others who may be still interested
Well, if some/many of chips investigated by Jim Millette are
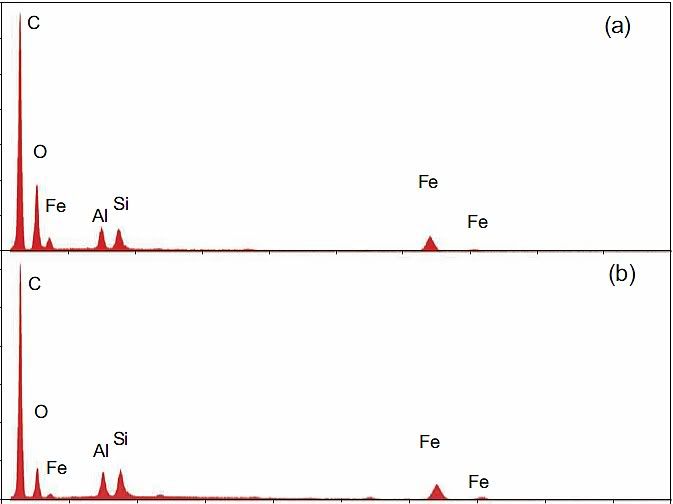
Laclede primer chips (as is indicated by their XEDS spectra and/or kaolinite pigment and/or epoxy resin, not speaking to iron oxide), they should contain ca 1.5 % of
strontium chromate. This anticorrosive stuff should have a form of
yellow crystalline needles with the lenghts of some micrometers or so. But, Jim has not found any strontium chromate, at least using his methods (XEDS, various microscopy).
(On the other hand, N. Harrit was so very kind and showed us a detailed XEDS spectrum of „Bentham chip a“, in which some strontium and chromium signals are detected – in his white paper WHY THE RED/GRAY CHIPS ARE NOT PRIMER PAINT.)
OK, it could not be easy to find pigment in such a low concentration in the paint, but is here any other explanation? Could strontium chromate crystals somehow „dissapear“ from the Laclede paint?
Yes, I think so (after some short study)

The matter is that up to now, I hadn' t any closer idea how this pigment could behave during electrocoating/curing/usage of Laclede primer paint, especially I have mostly overlooked passivation processes as important parts of anticorrosive action. In my head, there was only info that strontium chromate is very sparingly soluble in water.
Here is a short excerpt from the lengthy article
COATINGS FOR CORROSION CONTROL:
„The utility of chromate pigments for passivation is well established. Various mechanisms have been proposed to explain their effectiveness (38). All the proposed mechanisms require that the chromate ions be in aqueous solution. Like all passivators, chromate ions accelerate corrosion at low concentrations… Sodium dichromate is an effective passivating*agent, but would be a poor passivating pigment; its solubility in water is too high. It would be rapidly leached out of a film and would probably cause massive blistering. At the other extreme, lead chromate is so insoluble that it has no electrochemical action...
Strontium chromate (SrCrO4) has an appropriate solubility in water (5x10-3 mol CrO4/l) and is sometimes used in primers, especially latex paint primers…etc.“
This should mean that
strontium chromate needles in the freshly electrocoated paint are gradually
dissolved in the aqeous environment of the swollen paint, and they are probably further dissolved during curing and perhaps even later. Therefore, they could be basically missing in the paint, or they at least could change substantially their original form during this dissolution.
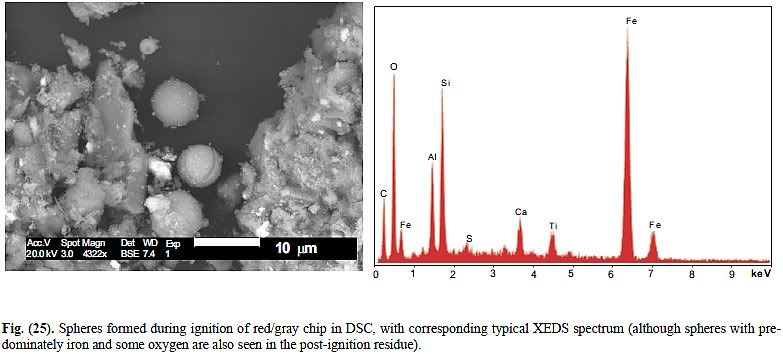
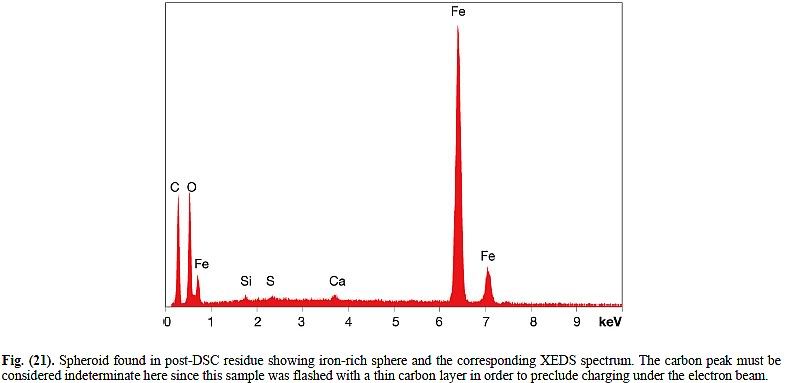
It seems that the fate of strontium and chromate ions („dissolved“ in water) in such wet paints is not really clear (I will add some good reference tomorrow), but chromate ions should travel to the painted steel and react with it forming very thin passivation layer. Therefore, at least chromium should not be easy to detect by XEDS, since it is "hidden" under the paint layer.
Btw, similar kind of passivation (migration of chromate ions to the protected steel and subsequent chemical reaction) is observed for zinc chromate (pigment in Tnemec primer), therefore even this stuff should be gradually dissolved in the wet paint to be effective „passivator“. The situation is more complex here, e.g. since various „zinc chromates“ are known, but any zinc chromate should be somehow chemically/physically transformed and this could shed a new light on the behavior of this pigment e.g. during the long term extraction of MEK chip with MEK.
Generally, the behavior of such passivating (and other) pigments in the electrocoated paint (a kind of colloid layer) can be quite complex and I have to admire researchers and engineers who have developed such sophisticated anticorrosive paints

)