Oystein
Penultimate Amazing
- Joined
- Dec 9, 2009
- Messages
- 18,903
I think I found a candidate LaClede primer chip:
It's the one identified as 9119-5230M3451B-crosssec2
Images in Appendix D
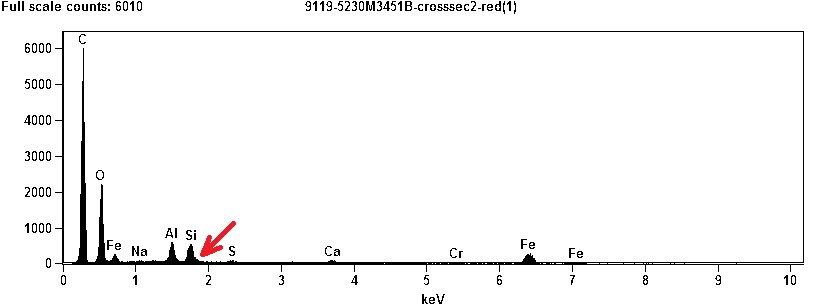
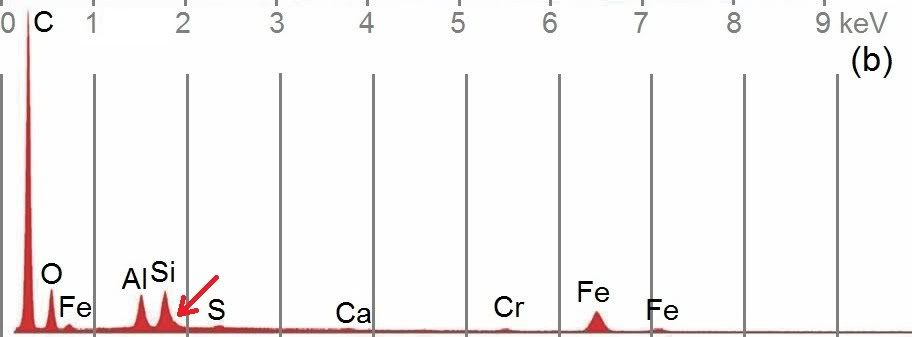
First, compare the red layer XEDS of this chips with Harrit's chip (b):
I notice in both:
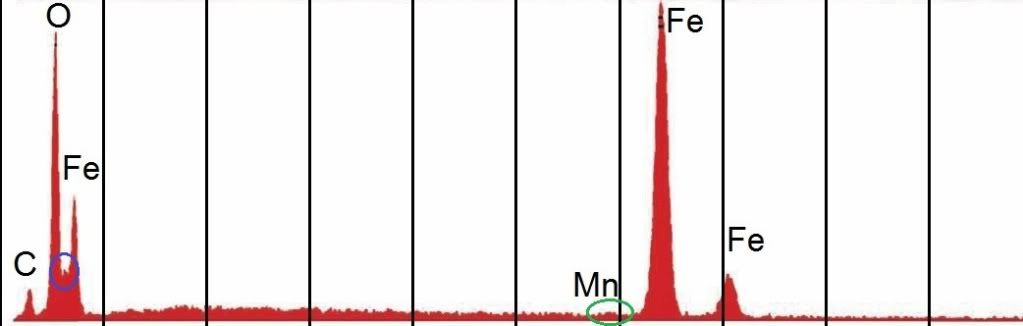
Now the gray layer XEDS:

I notice in both:
So both red and gray spectra are really nice matches.
And on to the image:

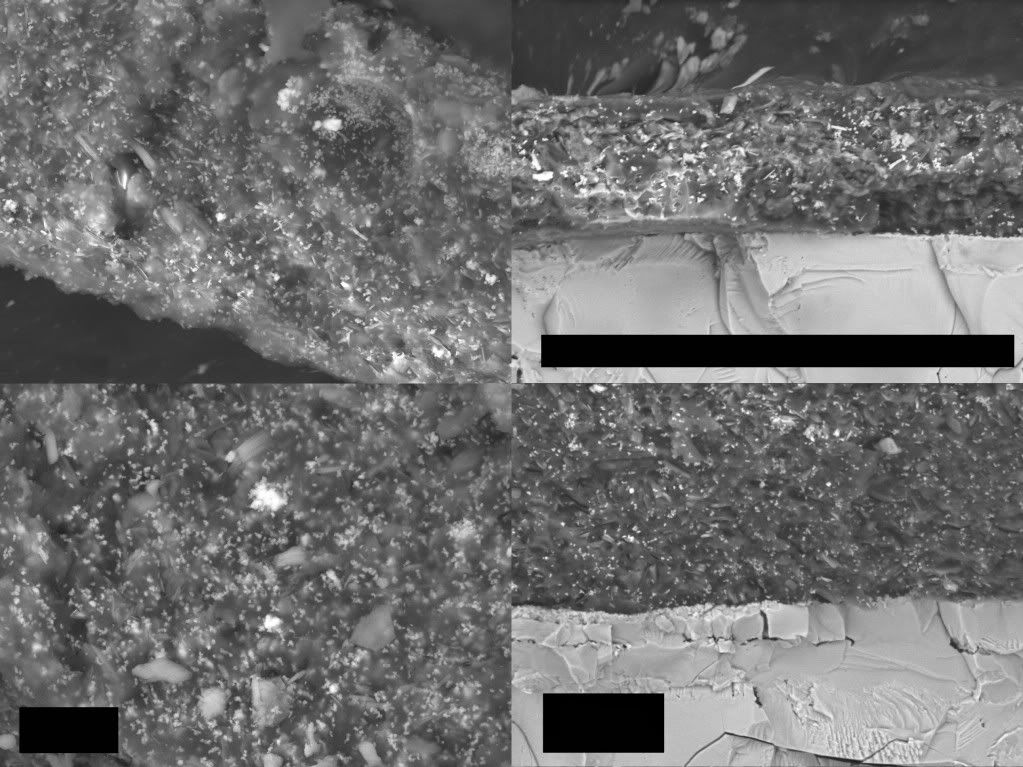
Is that BSE? Red layer is dark, gray layer bright. The gray layer is as homogeneous, brittle and edgy as the gray layers of Harrit. The red layer seems to be sprinkled with the 100nm hematite grains. Kaolin platelets are less than sharp, but the red arrows point at two acicular, whitish particles ca. 3µm long and perhaps 500nm thin. Candidates for Strontium Chromate?
What do you think, guys?
It's the one identified as 9119-5230M3451B-crosssec2
Images in Appendix D
First, compare the red layer XEDS of this chips with Harrit's chip (b):


I notice in both:
- C dominates far and away
- O is second highest (in Millette's spectrum, lighter elements are relatively stronger and heavier relatively weaker than in Harrit's. Such effects can come from geometry of the sample, attenuating factors, or would happen if Millette used a lower beam energy))
- Al and Si come next and are almost equal
- Si is "pregnant" on the right shoulder, at the 1.81 keV where Sr likes to hide!
- Traces of S, Ca and Cr
- And of course iron in fifth place
Now the gray layer XEDS:


I notice in both:
- Fe just a little higher than O
- There is this weird little peak between O and Fe L-alpha that doesn't correspond with any element's Edge energy as far as I can find, yet it's still there (also in chip c)
- I think there is a tiny signal for Mn near 5.9 keV, although neither Millette nir Harrit labeled it (I did)
- Little bit of C
So both red and gray spectra are really nice matches.
And on to the image:


Is that BSE? Red layer is dark, gray layer bright. The gray layer is as homogeneous, brittle and edgy as the gray layers of Harrit. The red layer seems to be sprinkled with the 100nm hematite grains. Kaolin platelets are less than sharp, but the red arrows point at two acicular, whitish particles ca. 3µm long and perhaps 500nm thin. Candidates for Strontium Chromate?
What do you think, guys?

