TheRedWorm
I AM the Red Worm!
- Joined
- Aug 13, 2007
- Messages
- 4,452
Fair enough. BTW, I don't understand a lot of it, but excellent work to all of the experts here.
What do you think the gray layer is Bill?Hi Ivan. Could it be said that the grey layer is more or less consistently the same thickness ?
Now you're just being mean. (it is fun watching him dig his hole deeper).What do you think the gray layer is Bill?
What do you think the gray layer is Bill?
Something like a skin you might say. Is the grey layer of consistent thickness over all the chips that you know of ?
Proceeding from the smallest to largest peaks, the yields are estimated to be approximately 1.5, 3, 6 and 7.5 kJ/g respectively. Variations in peak height as well as yield estimates are not surprising, since the mass used to determine the scale of the signal, shown in the DSC traces, included the mass of the gray layer. The gray layer was found to consist mostly of iron oxide so that it probably does not contribute to the exotherm, and yet this layer varies greatly in mass from chip to chip.
According to Harrit, no. In the Section about the DSC experiments of his Bentham article, he writes:
Source: The Open Chemical Physics Journal, 2009, 2, p. 19
It seems that this group has no clue about the exact constitution of the examined chips.
Thanks. Jones remarked that the grey layer is tough and seemed to stress that point. Would iron oxide be tough and grey ?
I then replied to this with:I remember Jones saying that the grey material was very tough. He seemed to stress that point but said no more about it. And I seem to remember somebody (maybe Jones at another point) speculating that the grey material might have been part of some kind of casing.
You Bill have now asked this:The word "tough" has a very specific meaning in materials science.When you look at the SEM photos of the gray layer it can't be "tough". Infact it's obvious it's a brittle material, the fractures ans the fracture surface indicate that - the exact opposite of toughness. Again it shows that Jones doesn't know what he's talking about.
This is why you fail Bill. You are profoundly incapable of learning. You refuse to acknowledge posts and answers directed to you and you ignore everything else that doesn't suit your fantasy and delusion, and then you just repeat what Jones has wrongly said.Thanks. Jones remarked that the grey layer is tough and seemed to stress that point. Would iron oxide be tough and grey ?
Something like a skin you might say. Is the grey layer of consistent thickness over all the chips that you know of ?
 A skin eh? And?. Is it the skin on a rice pudding? What is this skin you speak of? Why is there no detail in your answers? You then ask another question. Why are you asking these questions when a) you won't accept the answer and b) you don't understand the answer?
A skin eh? And?. Is it the skin on a rice pudding? What is this skin you speak of? Why is there no detail in your answers? You then ask another question. Why are you asking these questions when a) you won't accept the answer and b) you don't understand the answer?The chips are typically small but readily discernible by eye due to
their distinctive color. They are of variable size with major
dimensions of roughly 0.2 to 3 mm. Thicknesses vary from
roughly 10 to 100 microns for each layer (red and gray).
I now see he's managed to type the word gray into acrobe readers search box. Congratulations!
No it doesn't!'Tough' in technical terms means more or less what 'tough'means in general usage. Jones said that the grey layer is 'tough'.
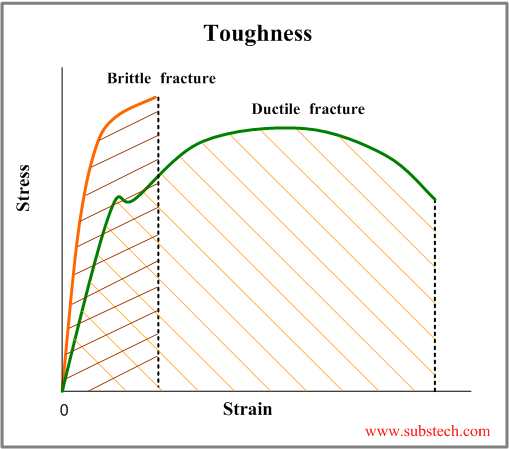

No. See above.He also said in the paper that it was composed of iron oxide,oxygen and a liitle carbon. Could that mixture be considered 'tough' in technical terms ?
No it doesn't!
Toughness, in materials science and metallurgy, is the resistance to fracture of a material when stressed. It is defined as the amount of energy per volume that a material can absorb before rupturing.
Toughness can be found by taking the area (i.e., by taking the integral) underneath the stress-strain curve.
[qimg]http://www.substech.com/dokuwiki/lib/exe/fetch.php?cache=cache&w=509&h=449&media=toughness.png[/qimg]
[qimg]http://www.substech.com/dokuwiki/lib/exe/detail.php?id=fracture_toughness&cache=cache&media=toughness.png[/qimg]
Right lets see if you can answer this question. Look at this photo.
[qimg]http://www.internationalskeptics.com/forums/picture.php?albumid=181&pictureid=872[/qimg]
Now using an arrow mark on the gray layer where you think plastic deformation has occured and say exactly why - what features of the fracture surface indicate plastic deformation? There must be plastic significant deformation for the material to be considered tough.
No. See above.
Now if you won't take my word for it then I suggest you take a degree in Materials Science so you can come to the same conclusion. There isn't a materials engineer on the planet that will say that material is tough. You have been on this site for 2 1/2 years - why have you not used that time to get a degree in a relevant subject to help you understand?
Surely....
I have just read an entry "Electrophoretic deposition" in Wiki. Basically, the steel is somehow cleaned/de-greased during the pretreatment, frequently with the formation of some very thin protective phosphate coating (can the phosphorus be detected by XEDS, since it has such a low atomic number (15)?)
You should specifically ask if the process would oxidize a surface layer of the steel....
My question to Sunstealer or other expert on metallurgy (or paint expert): can a comparatively high voltage applied during electrophoresis change the composition of the upper layer of the painted steel? (I mean could it somehow contribute to the composition/structure of the so-called gray layers in the chips?)
Thanks! If your colleague could provide a citation, that would be great, so we don't have to rely on hearsay!Paint aging: I have mentioned that I would not expect any substantial additional shrinkage (negative change of thickness) of the epoxide paint even during 30 years. But this is just my guess. Tomorrow, I will ask my colleague who had been working for some big Czech paint company for many years...
What was the reason again that Jones, Harrit, etc never got their samples sent to an independent lab?
Someone....truther...debunker...doesn't matter needs to press this issue with Jones....
Surely we can come up with a neutral lab to test the dust samples....I'm sure it can't be that expensive to get some XRD tests done on the samples along with various other tests....
At least then we can have some independent confirmation/verification of what this dust actually contains..
