Iron-rich does not mean "rich in
pure iron".
It means "rich in the iron element" but not necessarily uncombined.
http://www.google.com/search?q=iron-rich
http://www.google.com/search?q=iron-rich+-food
For example, from this hit (first hit of the second search which excludes the word "food"):
http://en.wikipedia.org/wiki/Iron-rich_sedimentary_rocks
Iron-rich sedimentary rocks are sedimentary rocks which contain 15% or more iron. [...] The main iron ores are from the oxide group consisting of hematite, goethite, and magnetite.
They are not called iron-oxide-rich. They are called iron-rich.
The ATM authors don't prove the iron is in elemental form in their spheres.
About figure 21, they say:
In the case of this iron-rich spheroid, the iron content exceeds the oxygen content by approximately a factor of two, so substantial elemental iron must be present.
However, they also previously said about the same figure:
Using back-scattered electron (BSE) imaging, spheres were selected in the post-DSC residue which appeared to be rich in iron. An example is shown in Fig. (21) along with the corresponding XEDS spectrum for this sphere.
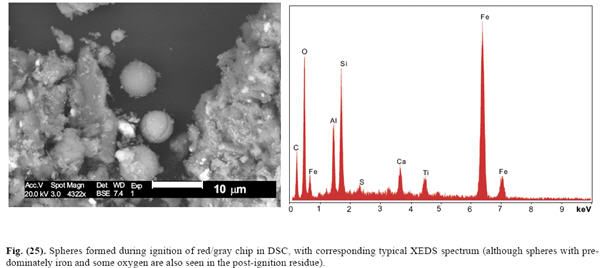
So, it was a selected example. Was it representative? Judging by figures 25 and 26 it wasn't. Those figure have enough oxygen as to account for all the iron, as well as other elements with which the iron could be combined. That's not a proof of elemental iron.
What did that sphere in figure 21 come from, then? Judging by Ivan's experiment using rusted paint, in which microspheres were formed that looked remarkably similar to those in the ATM paper, it could well have come from the gray layer (which Millette associates with rusted steel), as that would explain that spectrum. Or be some sort of contamination. The very same figure 21 clearly shows iron oxide crystals and kaolin platelets, as Sunstealer has repeatedly shown and no one has contended, so it's extremely unlikely that it comes from a reduction of the iron oxide particles because they're still there.