I think the batteries caused the Swiss cheese steel.
Drywall/plaster have a large sulphur content, and might well be the main source.
I think the batteries caused the Swiss cheese steel.
And how did the sulfur attack, exactly? Where did this "attacking sulfur" come from?
Molten steel is white or yellow. By the time it reaches red it is becoming a bit viscous.Is molten steel red-hot?
Drywall/plaster have a large sulphur content, and might well be the main source.
wikipedia said:Ignition of a thermite reaction normally requires only a simple child's sparkler or easily obtainable magnesium ribbon, but may require persistent efforts, as ignition can be unreliable and unpredictable. These temperatures cannot be reached with conventional black powder fuses, nitrocellulose rods, detonators, pyrotechnic initiators, or other common igniting substances. Even when the thermite is hot enough to glow bright red, it will not ignite as it must be at or near white-hot to initiate the reaction. It is possible to start the reaction using a propane torch if done correctly. The torch can preheat the entire pile of thermite which will make it explode instead of burning slowly when it finally reaches ignition temperature.
Not so much, ASTM A36 and A572 both melt at 2750 deg F. They were a large part of the rubble, right or wrong?
As for the eutectic 1800 deg F "liquid steel" mentioned in FEMA Appendix C, where did that attacking sulphur come from, exactly?
Smoldering fire doesn't need much oxygen. Several examples have been posted in this thread, from landfill fires lasting months, to an underground coal fire in Australia which burns at 1700°C at 1m / year and is estimated to be burning for 5,500 years. How do they get oxygen? Good question, perhaps by convection, similar to a thermal . But they do somehow.Fire needs oxygen, from air.
I think that Oystein was talking about energy density here.Which type of energy? Be specific.
Which means an energyEqn. 1: Fe2O3+ 2Al → 2Fe + Al2O3+ 181.5 kcal
Eqn. 2: 3Fe3O4+ 8Al → 9Fe + 4Al2O3+ 719.3 kcal
Melting temperture has nothing to do with corrosion resistance. And as far as steel grades go, A36 and A572 are on the low end of corrosion resistance.
Kind of reaching there, but it is far more plausible than thermite.
The batteries alone would supply more sulphuric acid than all the drywall would and at a far lower temperature. Heat that would liberate the sulphur from drywall would probably also release it from the pyrite inclusions that were found in the steel.
Not so much, ASTM A36 and A572 both melt at 2750 deg F. They were a large part of the rubble, right or wrong?
As for the eutectic 1800 deg F "liquid steel" mentioned in FEMA Appendix C, where did that attacking sulphur come from, exactly?
That's a lot of bravado Tom, bravo! But you still fail to answer the simple questions. Please do so, if you're able.
There are plenty of calculators on line that use the Structural Stability Research Council’s numerical methods, just plug in the geometry, materials and click the mouse button! Don't let your budding twoof-slaying fans down, they are cheering you on as I type.
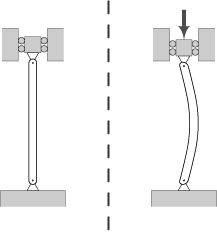
2a. How did no energy dissipate from the WTC 7 columns?
Explain this in terms of the Lagrangian energy theory. Tell me all about the dissipation term; please don't forget that ol' serpent in the garden.
He's absolutely correct. Sulphidation is driven by the process of surface diffusion, that is to say the diffusion of a species (in this case Sulphur) from the immediate atmosphere (most likely produced by the burning of material such as dry wall that contains Sulphur) into a solid's surface (in this case steel).Some catches for you -
2. Eutectic 'melting' is a different phenomenon from the gross phase-change that we normally refer to as 'melting'. The event here occurred along grain boundaries within the steel and would be more usefully described as corrosion or slow disintegration grain by grain. Had it been conventional melting there would be iron slag present and gross deformation of the entire section.
That you haven't yet grasped these simple facts speaks volumes for your belief-driven agenda.
Specualtion, but here are a few sources
Drywall
Plastics
foam seat cushions
Any one of those would give off a good amount of sulfur.
Are you done with the abstract yet Derek?
USGS aerial photos on 9-16-01 indicating 1340 deg F (712 deg C) is mighty, mighty strange.
You haven't even come up with a plausible explanation as to how the temperatures that USGS are possible. How is this possible? This isn't a wacky theory, it's just a simple question.
What process would take the sulfur from them and make it available for the reaction?
A very hot fire in an enclosed, damp place, e.g., a burning rubble pile.What process would take the sulfur from them and make it available for the reaction?
Let's call the central issue here "A".
"A" implies "B", which implies "C", which implies "D", and so on.
Derek's got us all arguing about "X".
Which is fine with him, as long as we stay as far away from "A" as possible.
(The actual meaning of "A", "B", "C", etc., is unimportant. It's the truther mode of thinking that keeps the discussion bogged down, regardless of the subject.)
